Research Andexer Lab
Chemoselective Methylation
The installation of the smallest carbon fragment – a methyl group – can change the chemical and physical properties of substrates in many ways. Therefore, methylation is of great interest for drug design and development. In industry, traditional methylation reagents such as methyl iodide are used. These chemicals are often toxic for humans and the environment. Additionally, they are highly reactive, increasing the number of side products. In nature, methyl groups are transferred onto substrates by so called methyltransferases (MTs). Those enzymes are highly chemoselective and can be divided into O- N- C- S- and halide MTs, depending on the (hetero) atom receiving the methyl group. Understanding the similarities and differences of these enzymes (structurally and mechanistically) will help us to establish environmentally friendly methods for methylation reactions.
Selected Publications:
How to Tell an N from an O: Controlling the Chemoselectivity of Methyltransferases
E. Jockmann, H. Girame, W. Steinchen, K. Kind, G. Bange, K. Tittmann, M. Müller, F. Feixas, M. Garcia-Borràs, J. N. Andexer
ACS Catal. 2025
Expanding the Substrate Scope of N- and O-Methyltransferases from Plants for Chemoselective Alkylation
E. Jockmann, F. Subrizi, M. K. F. Mohr, E. M. Carter, P. M. Hebecker, D. Popadić, H. C. Hailes, J. N. Andexer
ChemCatChem 2023
S-Methylation of Thiols Catalyzed by Different O-Methyltransferases
E. Abdelraheem, E. Jockmann, J. Li, S. Guenter, J. N. Andexer, P-L. Hagedoorn, U. Hanefeld
ChemCatChem 2023
S-Adenosylmethionine (Analog) Supply and Regeneration Systems
S-Adenosylmethionine (SAM) is a so-called group-transferring cofactor, or cosubstrate, meaning it is used stoichiometrically in each catalytic cycle. Consequently, cofactor regeneration or supply systems are necessary for using methyltransferases as biocatalysts. We are working on the implementation of such in vitro systems for different scenarios, including radical SAM enzymes and cofactor analogues.
Selected Publications:
Catalytic Alkylation Using a Cyclic S-Adenosylmethionine Regeneration System
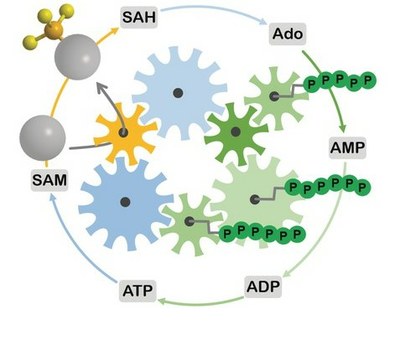
S. Mordhorst, J. Siegrist, M. Müller, M. Richter, J. N. Andexer
Angew. Chem. Int. Ed. 2017
This research article has been highlighted as Hot Paper in Biocatalysis.
Biomimetic S-Adenosylmethionine Regeneration Starting from Multiple Byproducts Enables Biocatalytic Alkylation with Radical SAM Enzymes
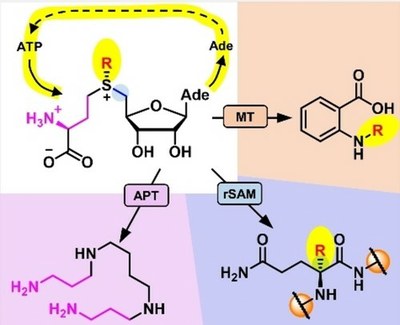
L. Gericke, D. Mhaindarkar, L. C. Karst, S. Jahn, M. Kuge, M. K. F. Mohr, J. Gagsteiger, N. V. Cornelissen, X. Wen, S. Mordhorst, H. J. Jessen, A. Rentmeister, F. P. Seebeck, G. Layer, C. Loenarz, J. N. Andexer
ChemBioChem 2023
Belonged to the 10% most read articles of the journal ChemBioChem in 2023!
Enzymatic Synthesis of L-Methionine Analogues and Application in a Methyltransferase Catalysed Alkylation Cascade
M. K. F. Mohr, R. Saleem-Batcha, N. V. Cornelissen, J. N. Andexer
A European Journal 2023
Rewiring Escherichia coli to transform formate into methyl groups
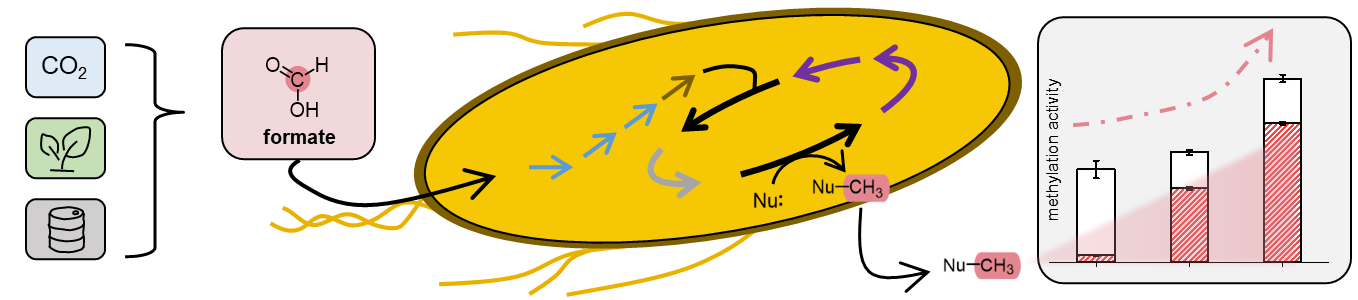
M. K. F. Mohr, A. Satanowski, S. N. Lindner, T. J. Erb, J. N. Andexer,
Microb. Cell Fact. 2025
A bicyclic S-adenosylmethionine regeneration system applicable with different nucleosides or nucleotides as cofactor building blocks.
D. Popadić, D. Mhaindarkar, M. H. N. Dang Thai, H. C. Hailes, S. Mordhorst, J. N. Andexer
RSC Chem. Biol. 2021
This research article was honored with the RSC „Outstanding Paper Award“.
Biocatalytic Synthesis and Regeneration of Nucleotides
We are using poyphosphate-dependent kinases for the regeneration of ATP and other nucleotides in our biomimetic cofactor regeneration cycles. In addition to this, we are exploring the suitability of these enzymes for the biocatalytic synthesis of (non-)physiological nucleotides.
Selected Publications:
Structural Insights into Broad-Range Polyphosphate Kinase 2-II Enzymes Applicable for Pyrimidine Nucleoside DiphosphateSynthesis
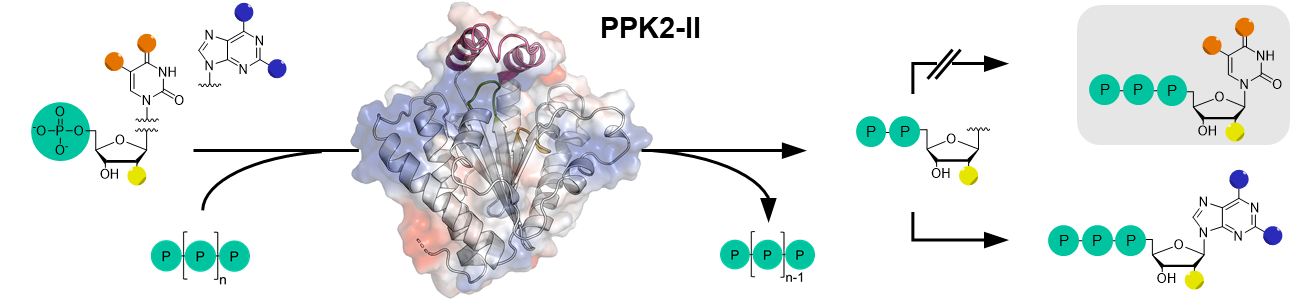
M. Kuge, M. Keppler, F. Friedrich, R. Saleem-Batcha, J. Winter, I. Prucker, P. Germer, S. Gerhardt, O. Einsle,
M. Jung, H. J. Jessen, J. N. Andexer
ChemBioChem 2025
This research article has been highlighted as VIP (Very Important Paper) in ChemBioChem.
Make or break: the thermodynamic equilibrium of polyphosphate kinase-catalysed reactions
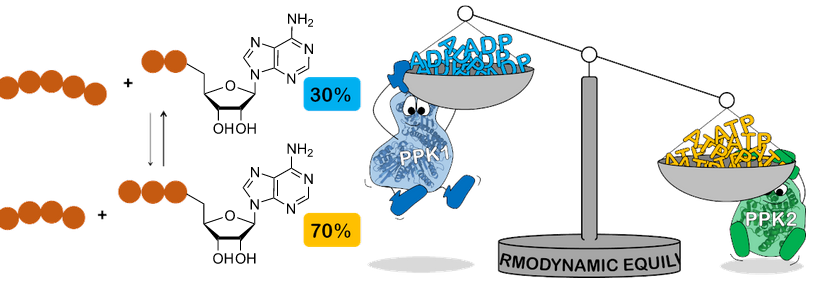
M. Keppler, S. Moser, H. J. Jessen, C. Held, J. N. Andexer
Beilstein J. Org. Chem. 2022
Non-canonical nucleosides: Biomimetic triphosphorylation, incorporation into mRNA and effects on translation and structure
P. Benčić, M. Keppler, M. Kuge, D. Qiu, L. M. Schütte, M. Häner, K. Strack, H. J. Jessen, J. N. Andexer, C. Loenarz
The FEBS Journal 2023
Review Articles
Selected Publications:
Biocatalytic One-Carbon Transfer – A Review
P. Germer, J. N. Andexer, M. Müller
Synthesis 2022, 54, 4401–4425 (review article)
Chorismate- and isochorismate converting enzymes: versatile catalysts acting on an important metabolic node
F. Hubrich, M. Müller, J. N. Andexer
Chem. Commun. 2021 (review article)
Round, round we go – strategies for enzymatic cofactor regeneration
S. Mordhorst, J. N. Andexer
Nat. Prod. Rep. 2020 (review article)

