Forschung AK Lüdeke
Chirality – a hallmark of life.
The building blocks of living organisms are small chiral molecules such as amino acids and sugars that may serve as precursors for biopolymers, primary or secondary metabolites, and in particular natural products. The individual chirality of the building blocks also induces higher order chirality when they assemble to larger entities (Figure 1). This supramolecular chirality is characterized by the asymmetric folding or braiding of a polymer, as in secondary or tertiary structure of proteins, or by the left- or right-handed helicity of higher-order aggregates formed from chiral subunits. Because of the asymmetry of their biopolymeric targets, many drug molecules are chiral, rendering chirality a highly important drug property. Furthermore, the chirality of biopolymers reflects their folding, functionality, or even pathogenicity such as in the supramolecular chirality of fibrillary aggregates.
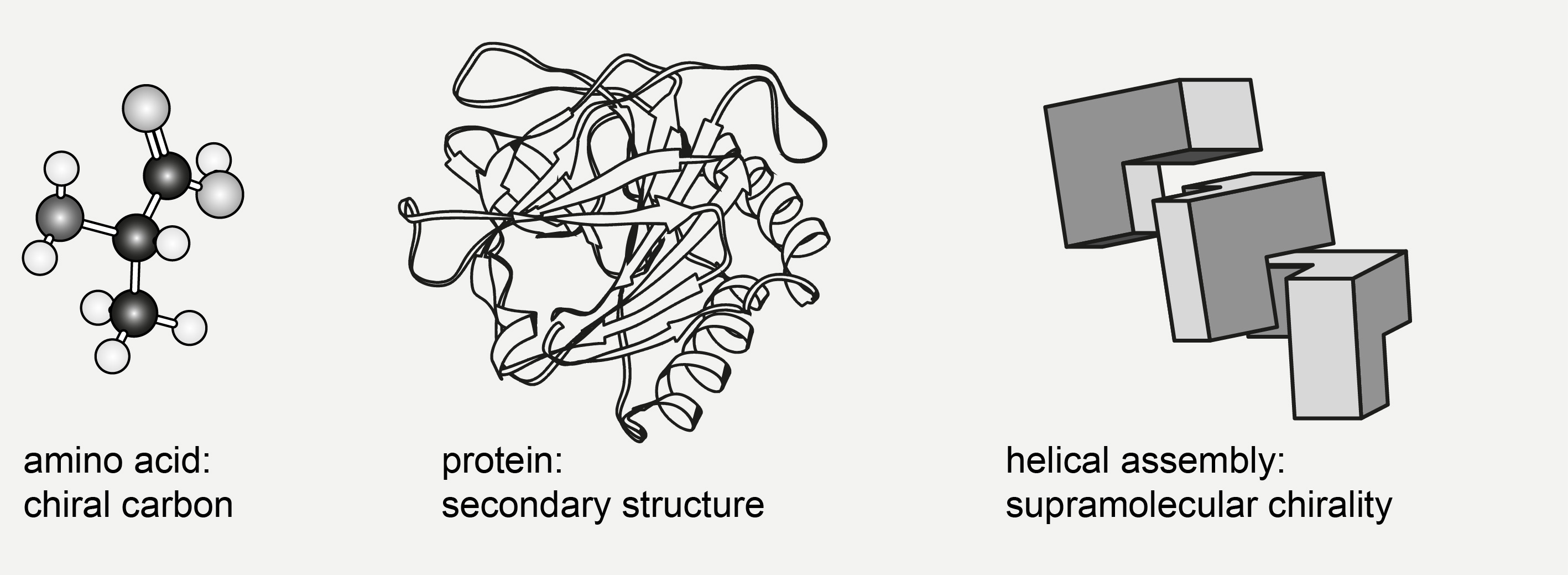
Figure 1. Different forms of molecular chirality.
By the use of circular dichroism (CD) and vibrational circular dichroism (VCD) we look at cues that induce chirality, be it in stereoselective enzymatic reactions, secondary structure, or the assembly of chiral subunits to larger chiral entities, with the aim to derive quantitative models that describe phenomena relevant in the context of life sciences.
Conformation: Secondary and higher-order structure.
The functionality of biopolymers is highly associated with their three-dimensional fold. X-ray crystallography provides us with valuable information about biopolymer structure. The question remains, whether what we see in these models is really the fold that is present in the physiological context. Higher-order structure may vary considerably in response to external parameters with consequences on the biological activity. We have found that the membranolytic activity of anti-cancer α-aminoxy-oligopeptides is likely associated with helical conformation (Figure 2) and that this structure is lost at high pH. Another example are biomineralization proteins, where a dynamic equilibrium between different conformations is involved in the precise control of the formation of biogenic minerals such as bone and enamel. This concept is not restricted to proteins, peptides and their analogs. We have shown that chemical modification of polysaccharides can lead to different secondary and higher-order structure. As these structural changes have consequences on the mechanical properties of hydrogels formed from these carbohydrate polymers it is possible to tailor gels being applicable for a potential use in regenerative medicine.
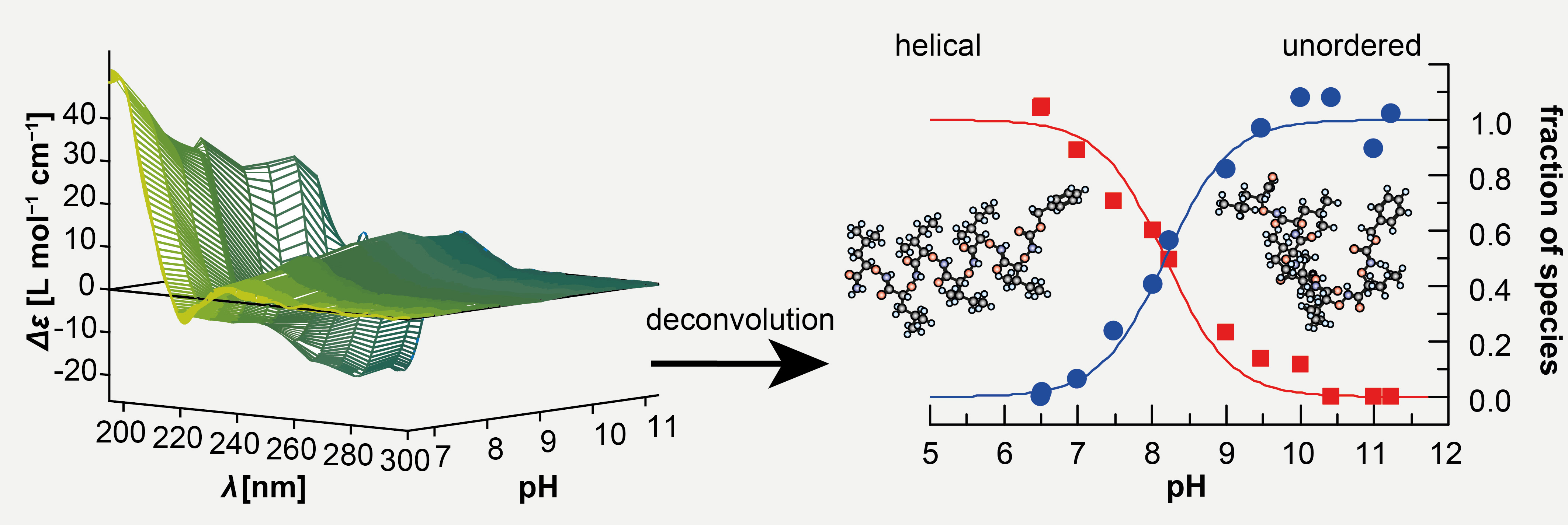
Figure 2. The equilibrium between helical and unordered α-aminoxy-oligopeptides is pH dependent.
Selected publications:
M. Sarem, S. Lüdeke, R. Thomann, P. Salavei, Z. Zou, W. Habraken, A. Masic, V. P. Shastri*: Disordered Conformation with Low PII Helix in Phosphoproteins Orchestrates Biomimetic Apatite Formation, Adv. Mater. 2017, DOI:10.1002/adma.201701629.
A. Rüther, A. Forget, A. Roy, C. Carballo, F. Mießmer, R. K. Dukor, L. A. Nafie, C. Johannessen, V. P. Shastri*, S. Lüdeke*: Unravelling a Direct Role for Polysaccharide β-Strands in the Higher Order Structure of Physical Hydrogels, Angew. Chem. Int. Ed. 2017, 56, 4603–4607
D. Diedrich, A. J. R. Moita, A. Rüther, B. Frieg, G. J. Reiss, A. Hoeppner, T. Kurz, H. Gohlke, S. Lüdeke, M. U. Kassack, F. K. Hansen*: α-Aminoxy Oligopeptides: Synthesis, Secondary Structure, and Cytotoxicity of a New Class of Anticancer Foldamers, Chem. Eur. J. 2016, 22, 17600–17611.
M. Sarem, S. Lüdeke*: Circular dichroism: A powerful tool for studying biomineralization promoter proteins. MRS Bull. 2015, 40, 490–498.
A. Forget, J. Christensen, S. Lüdeke, E. Kohler, S. Tobias, M. Matloubi, R. Thomann, V. P. Shastri*: Polysaccharide hydrogels with tunable stiffness and provasculogenic properties via a-helix to b-sheet switch in secondary structure, Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 12887–12892.
Supramolecular chirality: chiral complexes and aggregates.
Non-covalent self-assembly of smaller, chiral molecular entities may result in supramolecular chirality and is associated with neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease. In the case of fibrillary assembly, the smaller entities induce helicity in coiled and braided aggregates. The initial step is the formation of loosely bound dimers and every additional unit increases the stability of the aggregate. We employ weakly coordinated chiral-at-metal complexes of chiral ligands as a model to study the influence of environmental parameters on their spatial arrangement. The coordination geometry is indicated by the higher-order metal-centered chirality being evident by characteristic CD and VCD signals (Figure 3). In the case of proteins, the propensity for aggregation is associated with secondary structure. We have made similar observations for polysaccharides: preventing the formation of a double helix structure in agarose-derived hydrogels also prevents the formation of physical cross-links formed from multiple double-helices, which results in much softer gels.
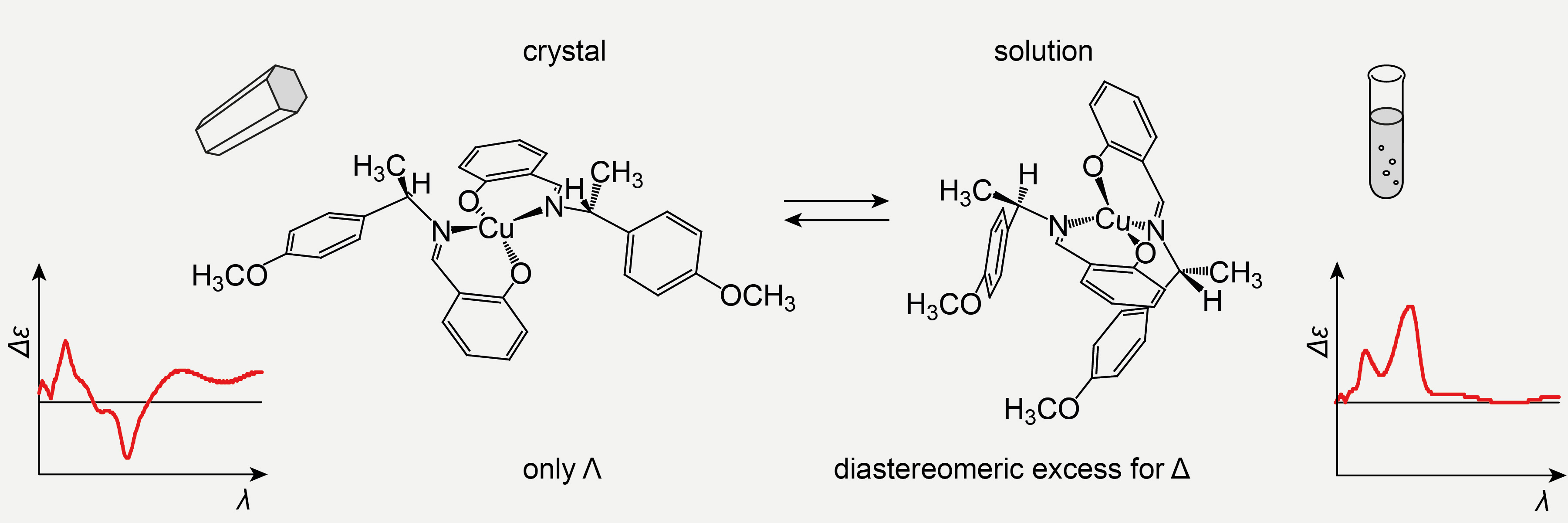
Figure 3. The helicity of a chiral-at-metal complex responds to changes in its environment (here: the solid and the solution state). The CD spectrum also changes considerably between the solid and the solution state.
Circular dichroism is particularly sensitive toward the monitoring of temperature-driven protein folding and assembly events. We are currently pursuing a project related to this effect with the aim to identify different stimuli that contribute to the self-assembly of elastin-like peptides.
Selected publications:
A. Rüther, A. Forget, A. Roy, C. Carballo, F. Mießmer, R. K. Dukor, L. A. Nafie, C. Johannessen, V. P. Shastri*, S. Lüdeke*: Unravelling a Direct Role for Polysaccharide β-Strands in the Higher Order Structure of Physical Hydrogels, Angew. Chem. Int. Ed. 2017, 56, 4603–4607.
A. C. Chamayou, G. Makhloufi, L. A. Nafie, C. Janiak, S. Lüdeke*: Solvation-Induced Helicity Inversion of Pseudotetrahedral Chiral Copper(II) Complexes. Inorg. Chem. 2015, 54, 2193–2203.
A. C. Chamayou, S. Lüdeke*, V. Brecht, T. B. Freedman, L. A. Nafie, C. Janiak*: Chirality and Diastereoselection of Δ/Λ-Configured Tetrahedral Zinc Complexes through Enantiopure Schiff Base Complexes: Combined Vibrational Circular Dichroism, Density Functional Theory, 1H NMR, and X-ray Structural Studies. Inorg. Chem. 2011, 50, 11363–11374.
Quantitative chiroptical spectroscopy: modeling dynamic processes
Inducing the reorganization of superstructure in biological macromolecules can be understood as a shift in the thermodynamic equilibrium between conformational states in response to an external stimulus. These structural changes are concomitant with spectral changes in the CD and the VCD (see above). Therefore, analyzing these signals as a probe for structural response allows establishing models that predict molecular structure as a function of, pH, concentration, temperature, etc. The analysis of the spectral data from monitoring by matrix-least-squares global fitting delivers the relative proportion of the species involved in the observed process. Furthermore, the global analysis also allows the deconvolution of the corresponding superimposed spectral contributions into interpretable spectra.
Particularly VCD is structurally sensitive and probes solution-state conformer populations. Aqueous solutions are generally too absorbent and commercial instruments cannot be used to measure such samples. Together with our collaborators, we developed a VCD spectrometer that uses a quantum cascade laser (QCL) as a light source and used this instrument for the monitoring of time- or pH-dependent processes (Figure 4).
Since its successful application on QCL-VCD data, we have also employed quantitative analyses of chiroptical data in the context of other projects (protein conformation, α-aminoxy peptides, polysaccharide higher-order structure, see above).
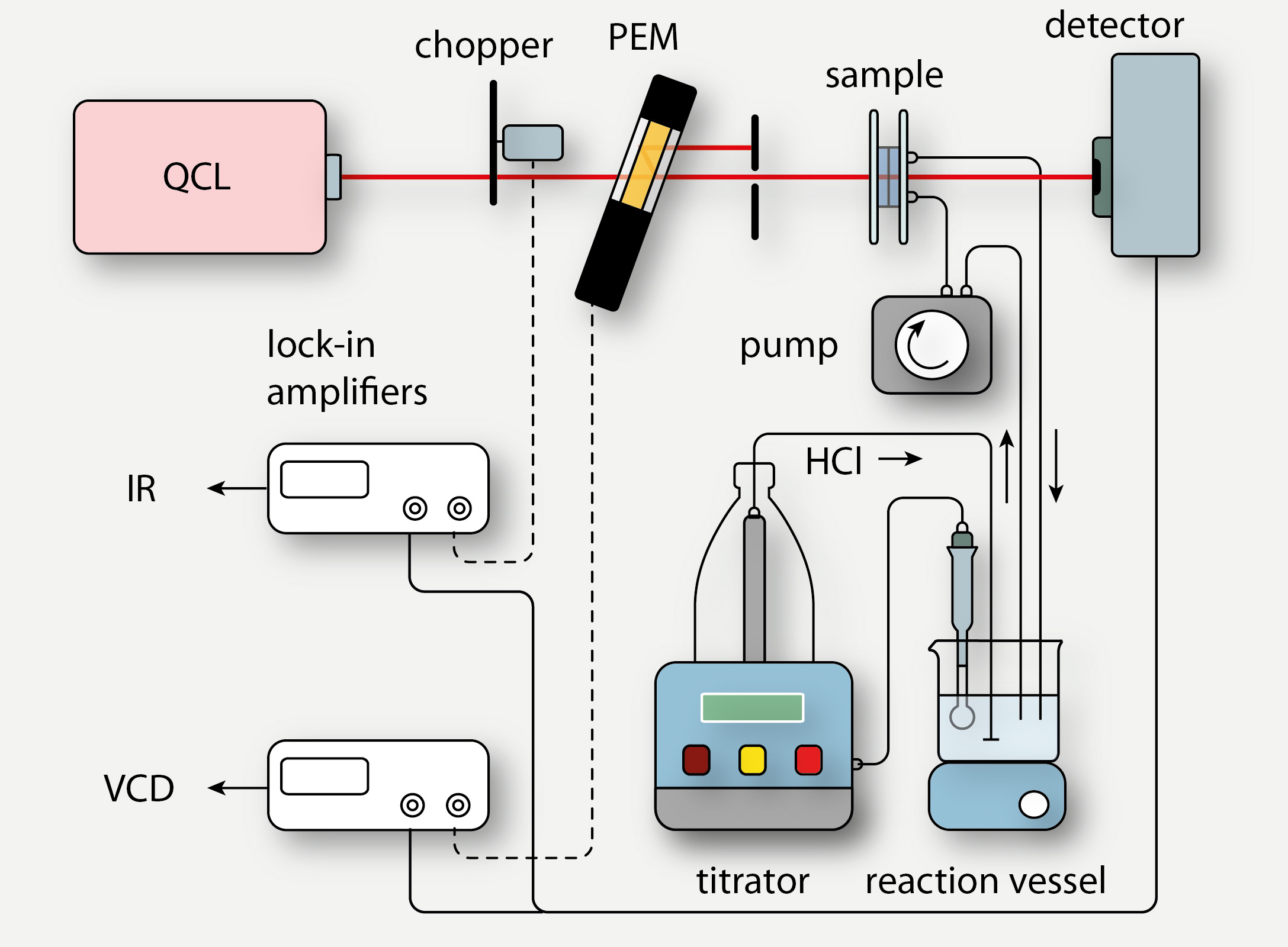
Figure 4. pH-Dependent proportion of anionic, zwitterionic, and cationic ʟ-proline determined from a matrix-least-squares global fit of a two-pK Henderson–Hasselbalch model to the pH-dependent spectra recorded by QCL-VCD.
Selected Publications:
Rüther, M. Pfeifer, V. A. Lórenz-Fonfría, S. Lüdeke: pH Titration Monitored by Quantum Cascade Laser-Based Vibrational Circular Dichroism, J. Phys. Chem. B 2014, 118, 3941–3949.
Rüther, M. Pfeifer, V. A. Lórenz-Fonfría, S. Lüdeke: Reaction monitoring using mid-infrared laser-based vibrational circular dichroism, Chirality 2014, 26, 490–496.
S. Lüdeke, M. Pfeifer, P. Fischer: Quantum-Cascade Laser-Based Vibrational Circular Dichroism, J. Am. Chem. Soc. 2011, 133, 5704–5707.
We are indebted to the Baden-Württemberg Stiftung for financial support of this research project through the Elite Programme for Postdoctoral Fellows.
Configuration: stereoselective enzymatic reactions
Natural products are often chiral and exhibit a high degree of structural complexity—attributes that are generally associated with biological activity. Responsible for the highly stereoselective biosynthesis of these compounds are enzymes that catalyze the crucial steps. Synthetic chemists make use of the outstanding stereoselectivity and –specificity of enzymes to get access to complex chiral molecules. The stereochemical outcome of the enzymatic conversion of an artificial substrate is not necessarily the same as what is observed for physiological substrates. We performed stereochemical analyses by VCD and other methods and observed considerable substrate-dependence of the stereoselectivity of enzymatic reactions suggesting that highly specific enzymatic reactivity is mediated by subtle substrate-enzyme interactions (Figure 5). The understanding of how substrate/enzyme interactions work is highly relevant for a potential use in chiral catalysis and provides insights into mechanisms of ligand/protein-interactions in general.
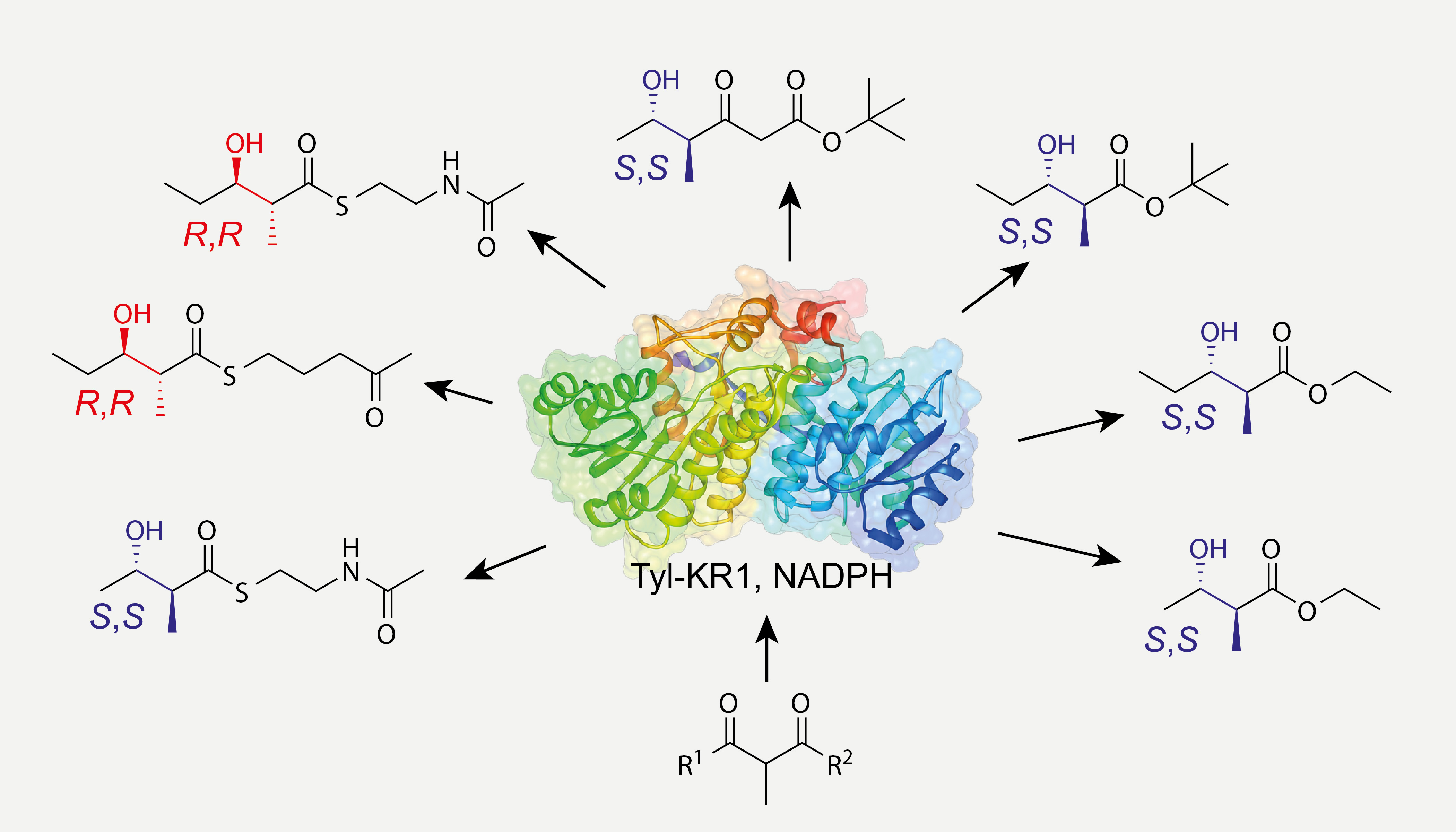
Figure 5.Depending on the substrate the keto reductase Tyl-KR1 delivers either R,R- or S,S-configured products. Interestingly, virtually no R,S- or S,R-diastereomers are observed.
As enantiomers of drug molecules or natural products might exhibit different biological activity it is crucial to determine the absolute configuration of a potential drug candidate. Particularly VCD-spectroscopy (in combination with quantum chemical calculations) is a straight-forward method for stereochemical assignments, in particular in cases where other methods such as x-ray crystallography, NMR or chiral phase chromatography fail.
Selected publications:
J. Haas, M. Häckh, V. Justus, M. Müller, S. Lüdeke*: Addition of a polyhistidine tag alters the regioselectivity of carbonyl reductase S1 from Candida magnoliae, Org. Biomol. Chem. 2017, 15, 10256–10264.
P. Lehwald, O. Fuchs, L. A. Nafie, M. Müller, S. Lüdeke*: Substrate-Determined Diastereoselectivity in an Enzymatic Carboligation. ChemBioChem 2016, 17, 1207–1210.
J. Haas, M. A. Schätzle, S. M. Husain, J. Schulz-Fincke, M. Jung, W. Hummel, M. Müller, S. Lüdeke*: A quinone mediator drives oxidations catalysed by alcohol dehydrogenase-containing cell lysates. Chem. Commun. 2016, 52, 5198–5201.
M. Häckh, M. Müller, S. Lüdeke*: Substrate-Dependent Stereospecificity of Tyl-KR1: An Isolated Polyketide Synthase Ketoreductase Domain from Streptomyces fradiae, Chem. Eur. J. 2013, 19, 8922–8928.

